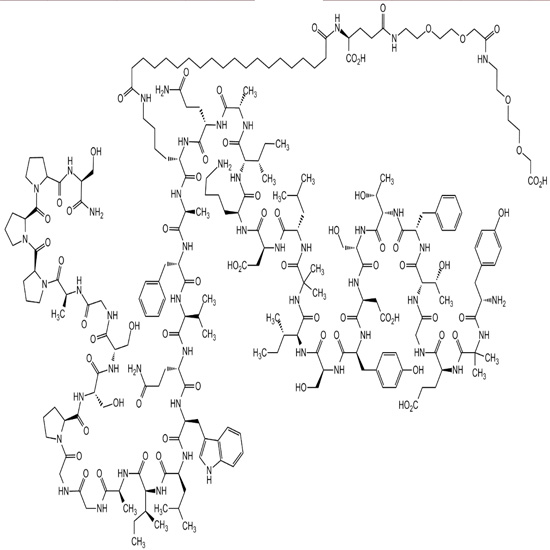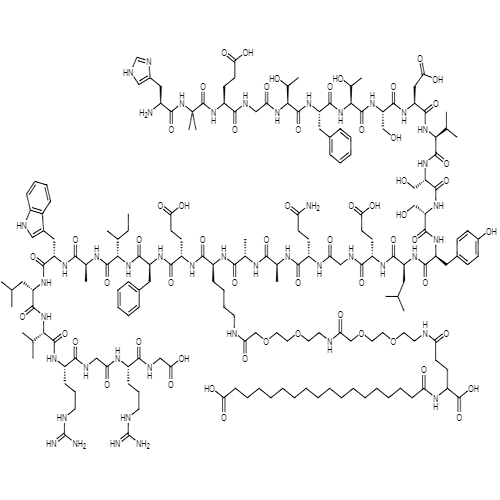

Semaglutide is a glucagon-like peptide-1 receptor agonist. The most common side effects include nausea, vomiting, diarrhea, abdominal pain, and constipation.
It was approved for medical use in the US in 2017. In 2021, it was the 90th most commonly prescribed medication in the United States, with more than 8 million prescriptions.
Semaglutide is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
The higher-dose formulation of semaglutide is indicated as an adjunct to diet and exercise for long-term weight management in adults with obesity (initial body mass index (BMI) ≥ 30 kg/m2) or who are overweight (initial BMI ≥ 27 kg/m2) and have at least one weight-related comorbidity.
In March 2024, the FDA expanded the indication for semaglutide (Wegovy), in combination with a reduced calorie diet and increased physical activity, to reduce the risk of cardiovascular death, heart attack and stroke in obese or overweight adults with cardiovascular disease.